
Elon Musk’s Neuralink to Launch Trial to Plug First-ever Implant into Human Brain
By Staff, Agencies
Neuralink, the neurotechnology company founded by Elon Musk that is building a brain-computer interface that can be implanted in the human brain - allowing bidirectional communication between the mind and external devices - received approval from the US Food and Drug Administration [FDA] in May to conduct its first human trials.
Elon Musk’s brain implant company, Neuralink Corp, has announced that it received approval from an independent review board to begin the first human trial of its brain implant for paralyzed patients.
"We are happy to announce that we’ve received approval from the reviewing independent institutional review board [FDA] and our first hospital site to begin recruitment for our first-in-human clinical trial,” Neuralink said in a statement on Tuesday.
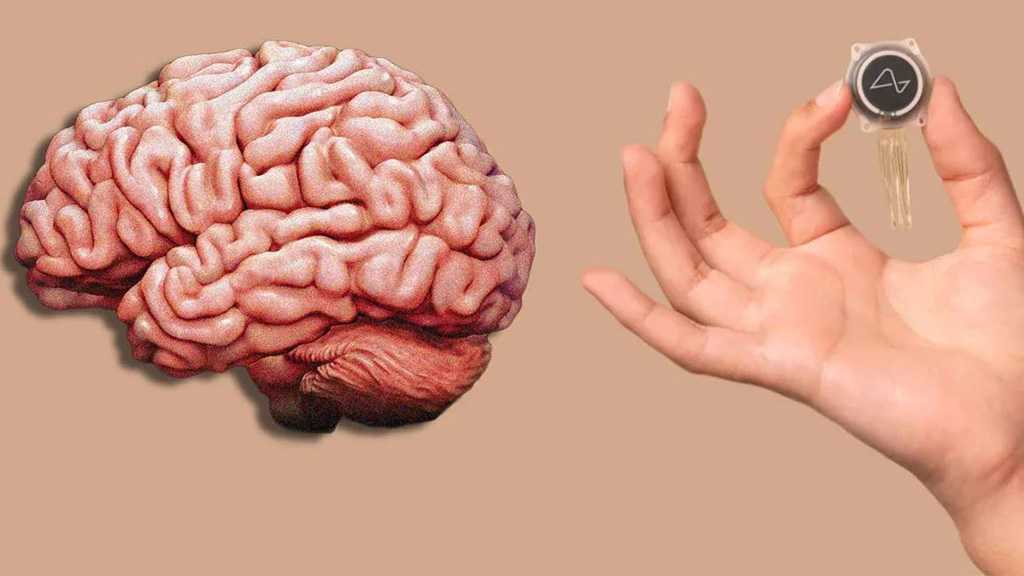
The brain-computer interface [BCI] company added that the “ground-breaking investigational medical device trial for our fully implantable, wireless BCI aims to evaluate the safety of our implant and surgical robot, and assess the initial functionality of our BCI for enabling people with paralysis to control external devices with their thoughts.”
As for who can qualify for the six-year study, Neuralink is seeking out patients with paralysis caused by cervical spinal cord injury or amyotrophic lateral sclerosis [ALS]. Participants recruited for the trial will have a proprietary robot surgically implant the BCI in a region of their brain that controls movement.
Accordingly, it is hoped that the device will enable them to control a keyboard or the computer cursor by the power of their thoughts.
The purpose of the trial is to test the safety and efficacy of Neuralink's implantable BCI, called “the Link,” which is a surgically embedded neural-chip implant designed to decode and stimulate brain activity.
Neuralink's announcement comes after it got regulatory approval from the FDA in May for its first human trials.
If successful, the cutting-edge technology developed by Neuralink could revolutionize various fields, including healthcare, communication, and human-computer interaction, with potential applications ranging from helping individuals with neurodegenerative disorders, such as Parkinson's or Alzheimer's disease, to enhancing cognitive abilities and enabling novel methods of information processing.
However, the company has faced trials of its own along the way: its previous testing in animals prompted a great deal of scrutiny after reports that trials were causing unnecessary suffering, and the death of 1,500 animals between 2018 and 2022, according to reports. It was also claimed that all sorts of human errors had occurred because of the company’s impatience to reach its ambitious goals.
If Neuralink's device proves safe for human use, it could, nevertheless, take decades before it is cleared for patients outside the realm of clinical trials, experts have emphasized.
Comments
- Related News

FT: World Gripped by Mental Health Pandemic
24 days ago

