
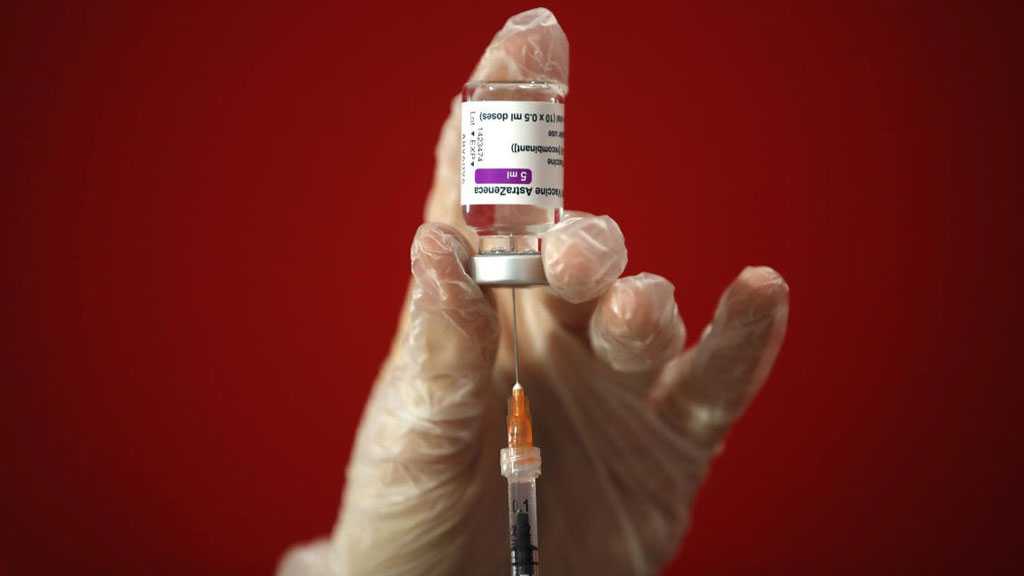
EU Drug Regulator Says AstraZeneca Vaccine Safe, Blood Clotting Link Not Ruled Out with Certainty
By Staff, Agencies
The European Medicines Agency said on Thursday that it considers the COVID-19 coronavirus vaccine by AstraZeneca and the Oxford University to be safe for use.
However, the body also said it was unable to rule out with certainty a link between the vaccine and rare blood clotting cases, including several fatal ones.
The concerns on the matter have prompted a range of EU countries, including Germany and France, to suspend the use of the vaccine; following the announcement, a number of EU states announced that the use of the jab will now resume.
Earlier in the day, UK's drugs regulator said its own review did not find evidence directly linking the jab with blood clotting.
At the same time, Norwegian media on Thursday cited a group of scholars with Rikshospitalet, Oslo University Hospital, as saying the vaccine was likely what triggered the thrombosis, adding it set off an excessive immune response.
The assessment was based on the records of three health workers who had been admitted into the hospital with the rare thrombosis, with one of them dying.
Holme said the vaccine was the likeliest suspect for triggering the immune response as the patients', all of them aged under 50, health records, offer no clues on anything else that could have set off the condition.
Comments
- Related News



