Human Protein Found to Make Coronavirus Highly Infectious
By Staff, Agencies
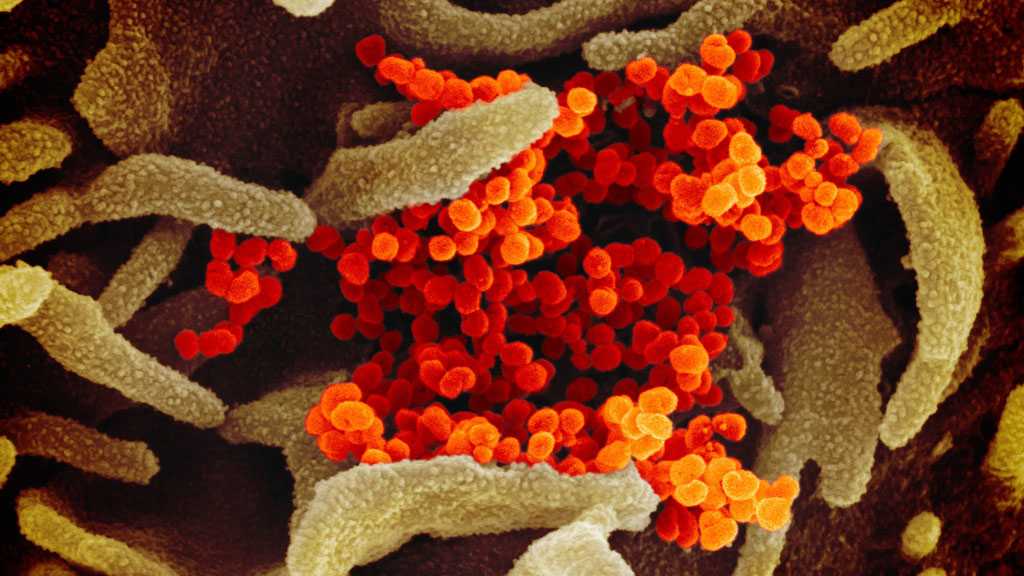
A protein on the surface of human cells is believed to be responsible for making coronavirus highly infectious, according to British scientists.
Neuropilin-1 is thought to accelerate the speed of transmission of Sars-CoV-2, the virus that causes COVID-19, between people, The Independent reported.
Researchers from University of Bristol said their findings, published in the journal Science, could help in developing antiviral treatments that block the “newly discovered interaction between virus and host.”
"To defeat COVID-19 we will be relying on an effective vaccine and an arsenal of anti-viral therapeutics,” the team added.
"Our discovery of the binding of the Sars-CoV-2 Spike to neuropilin-1 and its importance for viral infectivity provides a previously unrecognized avenue for anti-viral therapies to curb the current COVID-19 pandemic."
Sars-CoV-2 is believed to infect humans by first attaching itself to the surface of human cells that line the respiratory tract, using what is known as the "spike" protein.
Once attached, the virus invades the cell and makes multiple copies of itself which are then released into the body, causing COVID-19.
The researchers added that understanding the process by which the virus's spike protein recognizes human cells is "central to the development of antiviral therapies and vaccines to treat COVID-19".
They said: "In looking at the sequence of the Sars-CoV-2 spike protein we were struck by the presence of a small sequence of amino acids that appeared to mimic a protein sequence found in human proteins which interact with neuropilin-1.
"This led us to propose a simple hypothesis: could the spike protein of Sars-CoV-2 associate with neuropilin-1 to aid viral infection of human cells?”
"Excitingly, in applying a range of structural and biochemical approaches we have been able to establish that the spike protein of Sars-CoV-2 does indeed bind to neuropilin-1,” the researches said.
"We were able to show that the interaction serves to enhance Sars-CoV-2 invasion of human cells grown in cell culture."
The team said that, by using monoclonal antibodies, which are lab-created proteins that resemble naturally occurring antibodies, or a drug that blocks the interaction of neuropilin-1 with the coronavirus, they were able to reduce the virus's ability to infect human cells.
They added: "This serves to highlight the potential therapeutic value of our discovery in the fight against COVID-19."
Vaccines, in contrast, work by training the human immune system to recognize Sars-CoV-2’s spike protein and produce the antibodies needed to defend against it.
Prof Lawrence Young, a professor of molecular oncology at Warwick Medical School who was not involved in the study, described it as “another ‘brick in the wall’ of our understanding of the biology of SARS-CoV-2 infection”.
He said it “contributes to an improved understanding of the way the virus infects cells” and “will help us as we think about the future of vaccine development and possible anti-viral drugs”.
Comments
- Related News